Viral Vectors and Plasmid DNA Manufacturing Market Size Worth USD 26.66 Billion by 2034 Driven by Gene Therapy Demand
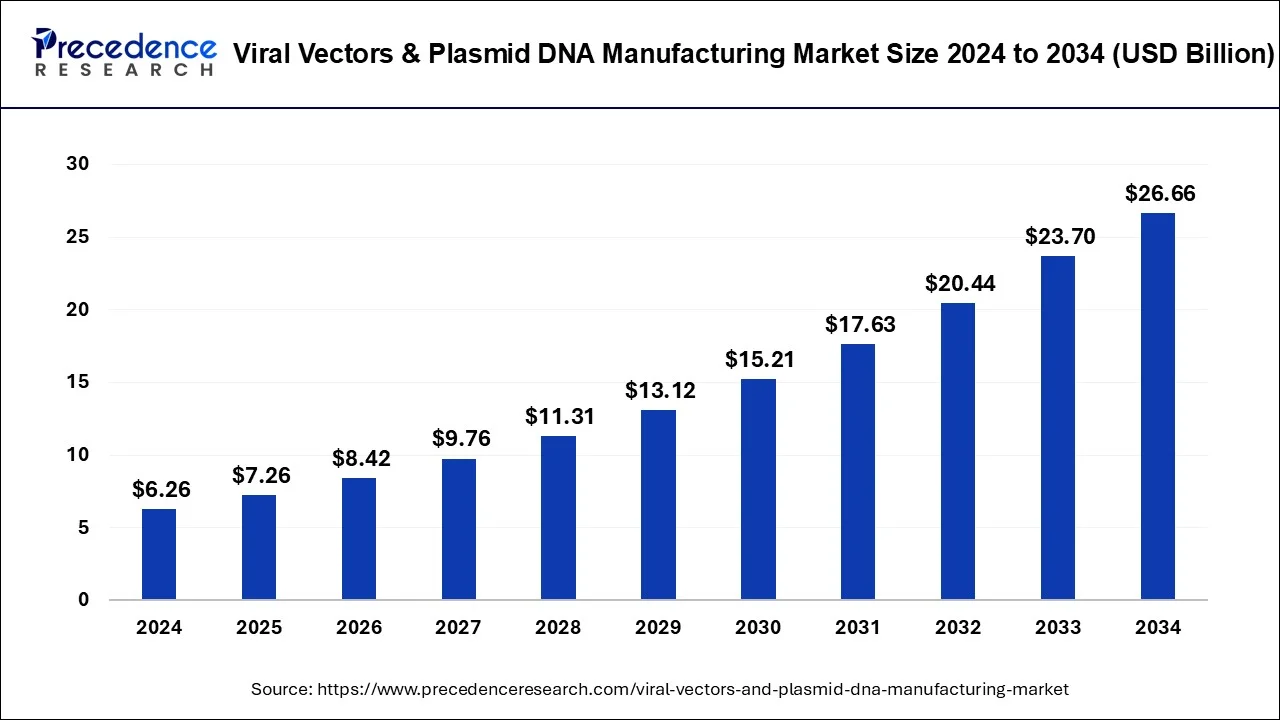
The global viral vectors and plasmid DNA manufacturing market size is expected to be worth USD 26.66 billion by 2034 increasing from USD 7.26 billion in 2025 and is representing a double-digit CAGR of 15.59% from 2025 to 2034.
Ottawa, Aug. 21, 2025 (GLOBE NEWSWIRE) -- The global viral vectors and plasmid DNA manufacturing market size surpassed USD 6.26 billion in 2024 and is estimated to grow from USD 7.26 billion in 2025 to around USD 26.66 billion by 2034. The global market is expected to expand at a strong CAGR of 15.59% from 2025 to 2034. The viral vectors and plasmid DNA manufacturing market is driven by the surging demand for gene therapies, vaccines, and cell-based treatments.
Note: This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.
Try Before You Buy – Get the Sample Report@ https://www.precedenceresearch.com/sample/1012
Viral Vectors and Plasmid DNA Manufacturing Market Overview
What is the Viral Vectors and Plasmid DNA Manufacturing Market?
The viral vectors and plasmid DNA manufacturing market is a fast-growing niche in the biopharmaceutical sector as driven by the rapidly growing demand for advanced genetic therapies, vaccines, and novel drug delivery systems.
In transduction, an efficient delivery of genetic material into host cells with the use of viral vectors is usually done using viral vectors like adenoviruses, including lentivirus and herpes simplex virus. Improvements in molecular biology, increased investments in gene therapy, and the explosion in clinical trials related to genetic medicines are seen as the key growth drivers in this market.
Key Takeaways: Viral Vectors & Plasmid DNA Manufacturing Market
- In terms of revenue, the global viral vectors and plasmid DNA manufacturing market is predicted to touch USD 8.42 billion in 2026.
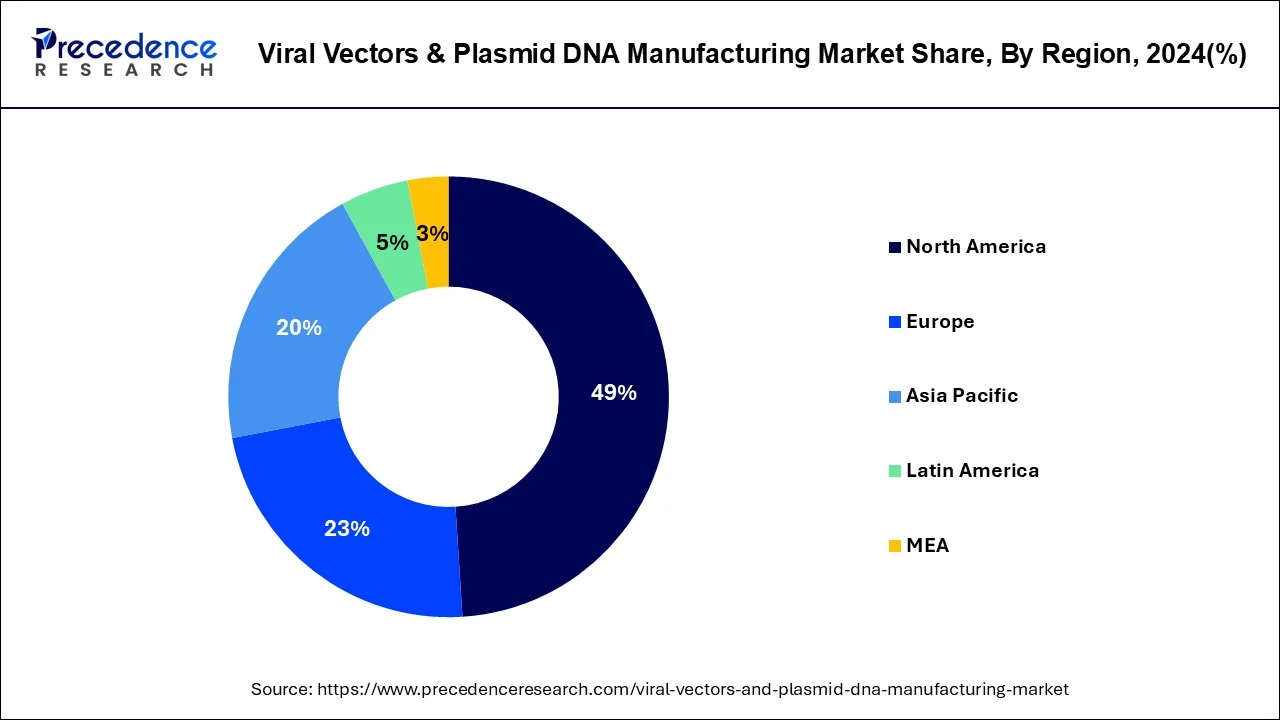
- North America accounted for the largest market share of 49% in 2024.
- By vector type, the AAV segment contributed the highest market share of 21% in 2024.
- By workflow, the downstream processing segment held the major market share of 54% in 2024.
- By application, the vaccinology segment captured the largest market share of 22.5% in 2024.
- By disease, the cancer segment dominated the market with the biggest market share of 38% in 2024.
- By end use, the research institutes segment generated the highest market share of 58.4% in 2024.
Viral Vectors and Plasmid DNA Manufacturing Key Trends:
-
Growth in Gene & Cell Therapy Pipeline
- Increasing approvals of gene therapies for rare diseases and cancers.
- High demand for AAV, lentivirus, and plasmid DNA as therapeutic delivery tools.
-
Advancements in Manufacturing Technologies
- Adoption of single-use bioreactors and modular manufacturing systems.
- Shift to serum-free and chemically defined media.
- Automation and artificial intelligence (AI) for process optimization and higher yields.
-
Use of digital twins and real-time monitoring for smarter production.
-
Outsourcing and CDMO Expansion
- Surge in outsourcing to contract development and manufacturing organizations (CDMOs).
- Significant investment in new manufacturing capacity by major players
- CDMOs becoming critical for scalability and speed to market.
-
Geographic Market Expansion
- Rapid growth in the Asia-Pacific region due to supportive regulation and infrastructure.
➤ Get the Full Report @ https://www.precedenceresearch.com/viral-vectors-and-plasmid-dna-manufacturing-market
Viral Vectors and Plasmid DNA Manufacturing Market Opportunity
Advancements in Gene Therapies:
The use of viral vectors is becoming a key therapy in the delivery of genetic programs directly to the target cells, so therapies can reach the causes of genetic disorders, cancers, and rare diseases. As gene therapies gain more acceptance by regulatory agencies and other healthcare systems, there has been a current surge in demand for high-quality viral vectors and plasmid DNA. There are also scalable, efficient, and cost-effective production opportunities developing with the ongoing integration of automation and bioreactor technologies and advanced analytics.
Viral Vectors and Plasmid DNA Manufacturing Market Challenge:
Regulatory compliance remains the biggest challenge in the viral vectors and plasmid DNA manufacturing market. To ensure patient safety, quality, and enforce strict standards, regulatory agencies, like the FDA (U.S. Food and Drug Administration), EMA (European Medicines Agency), and others, scrutinize the manufacturing processes and pre-clinical and clinical trial processes to lineup with their criteria. In addition, the scalability of viral vector and plasmid DNA manufacturing, which ensures consistency and conformance to regulatory requirements, is a technological challenge.
Case Study: Scalable Manufacturing of AAV Vectors for Rare Disease Therapy

In 2024, a leading U.S.-based biotech company partnered with a contract development and manufacturing organization (CDMO) to accelerate the clinical production of adeno-associated virus (AAV) vectors for a rare genetic disorder. By integrating single-use bioreactors, serum-free media, and automated downstream processing, the CDMO was able to reduce production time by 40% while ensuring regulatory compliance under FDA standards.
This collaboration not only enabled faster progression to Phase II clinical trials but also highlighted the crucial role of outsourced manufacturing capacity in meeting the rising demand for gene and cell therapies. The case demonstrates how CDMOs are becoming pivotal in bridging innovation and large-scale commercialization in the viral vectors and plasmid DNA manufacturing market.
Scope of Viral Vectors and Plasmid DNA Manufacturing Market
| Report Attributes | Key Statistics |
| Market Size in 2024 | USD 6.26 billion |
| Market Size in 2025 | USD 7.26 billion |
| Market Size in 2031 | USD 17.63 billion |
| Market Size by 2034 | USD 26.66 billion |
| Growth Rate 2025 to 2034 | CAGR of 15.59% |
| Leading Region in 2024 | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Vector Type, Application, Workflow, End-User, Disease, and Regions |
| Regional Scope | Asia Pacific, North America, Europe, Latin America, Middle East and Africa |
➡️ Become a valued research partner with us ☎ https://www.precedenceresearch.com/schedule-meeting
Viral Vectors and Plasmid DNA Manufacturing Market Key Regional Analysis:
What is the Market Size of the U.S. Viral Vectors and Plasmid DNA Manufacturing Market?
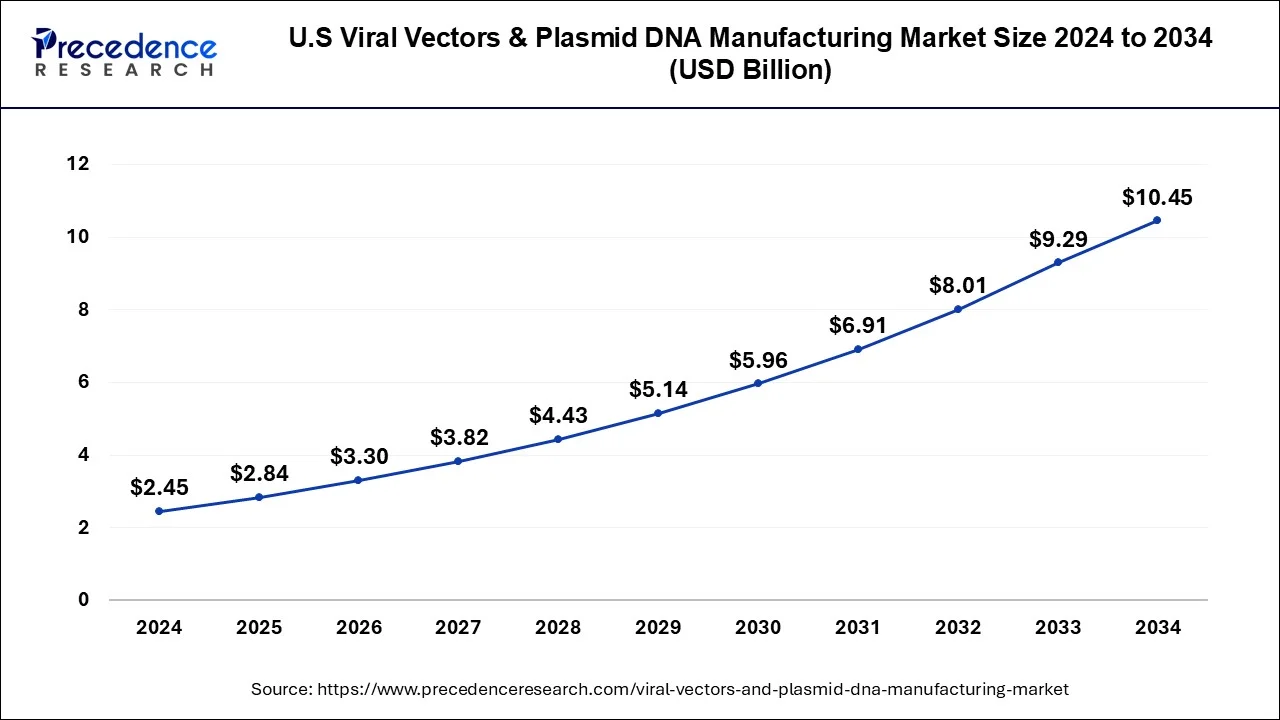
According to Precedence Research, the U.S. viral vectors and plasmid DNA manufacturing market size reached USD 2.45 billion in 2024 and is expected to rise from USD 2.84 billion in 2025 to approximately USD 10.45 billion by 2034 with a CAGR of 15.61% from 2025 to 2034.
The Complete Study is Immediately Accessible | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/1012
How North America Dominated the Viral Vectors and Plasmid DNA Manufacturing Market?
North America dominated the viral vectors and plasmid DNA manufacturing market in 2024, due to its infrastructure that makes the sophisticated healthcare movable, a healthy biotechnology ecosystem, and clinical research leadership.
The existence of a strong regulatory environment where proper guidance is given by the FDA lends comfort to manufacturers and expedites the clinical trial process to facilitate faster commercialization. This is also due to the increased use of innovative manufacturing technologies, including automation and advanced bioreactors, which guarantee production scalability and efficiency.
Why is Asia-Pacific the Fastest-Growing in the Viral Vectors and Plasmid DNA manufacturing Market?
Asia-Pacific experiences the fastest growth in the market during the forecast period. Emerging markets like China, India, Japan, and South Korea have been spending a lot in the field of biotechnology, developing dedicated research centers, and also building on their clinical trial system. This has given a prosperous opportunity to biotechnology startups as well as large pharmaceutical firms to explore their facilities in the production of viral vectors and plasmid DNA. Increasing cases of cancer and genetic disorders, as well as chronic diseases in the area, further contribute to demand for more sophisticated therapies, driving the need to have a manufacturing capacity.
Viral Vectors and Plasmid DNA Manufacturing Market Segmentation Analysis
Vector Type Analysis
The adenovirus segment dominated the viral vectors and plasmid DNA manufacturing market in 2024. The AAV vectors are non-pathogenic with low immunogenicity that can infect both dividing cells and non-dividing cells, thus making them a priori for producing therapy against genetic diseases. The clinical success and commercialization potential of AAV are evidenced in several FDA- and EMA-approved treatments, such as Luxturna to treat inherited retinal dystrophy and Zolgensma to treat spinal muscular atrophy. Further, the scalable production process and a robust regulatory environment have consolidated the control of AAV.
The lentivirus segment is the fastest-growing in the market during the forecast period. Lentiviruses integrate into the host genome, guaranteeing long-term and stable expression of the gene, which is essential to the treatment of chronic and inherited diseases. Notably, lentiviruses have a higher gene capacity than AAV, thus being more appropriate to deliver complex genes. Increased clinical testing, a broader scope of therapeutics, and the increasing alliance of therapeutic biotechnology firms and CDMOs are driving the uptake of scalable, lentiviral manufacturing.
Application Analysis
The vaccinology segment held the largest share in the viral vectors and plasmid DNA manufacturing market in 2024. The pandemic also amplified investments into vaccine technology across the globe, and the benefits of using viral vectors and plasmid DNA compared to traditional platforms. Such modern technologies allowed accelerating the development process, high safety rates, and large-scale production, which led to the record speed of the availability of vaccines on the market. With pandemic preparedness and response to emerging infectious diseases remaining of top concern to governments, research institutions, and pharmaceutical companies.
The cell therapy segment experiences the fastest growth in the market during the forecast period. Viral vectors are important in cell therapy gene delivery, as accurate genetic alterations prevent the patient-derived cells from fighting the diseases. The field of gene-editing technologies like CRISPR is improving the therapeutic potential of viral vector-based cell therapies, expanding their areas of use. The cell therapy segment will grow faster than any other, with strong pipelines in oncology, rare genetic disease, and regenerative medicine.
Workflow Analysis
The upstream processing segment dominated the viral vectors and plasmid DNA manufacturing market in 2024. As more gene therapies are approved for the market and there is an increasing need for scalable production platforms, upstream processing has taken central stage in creating a sustainable supply to downstream workflows. Its capability to provide stable raw material production is of key importance towards sustaining growing pipelines in advanced therapies, and stabilized quality and high volumes in production in industries.
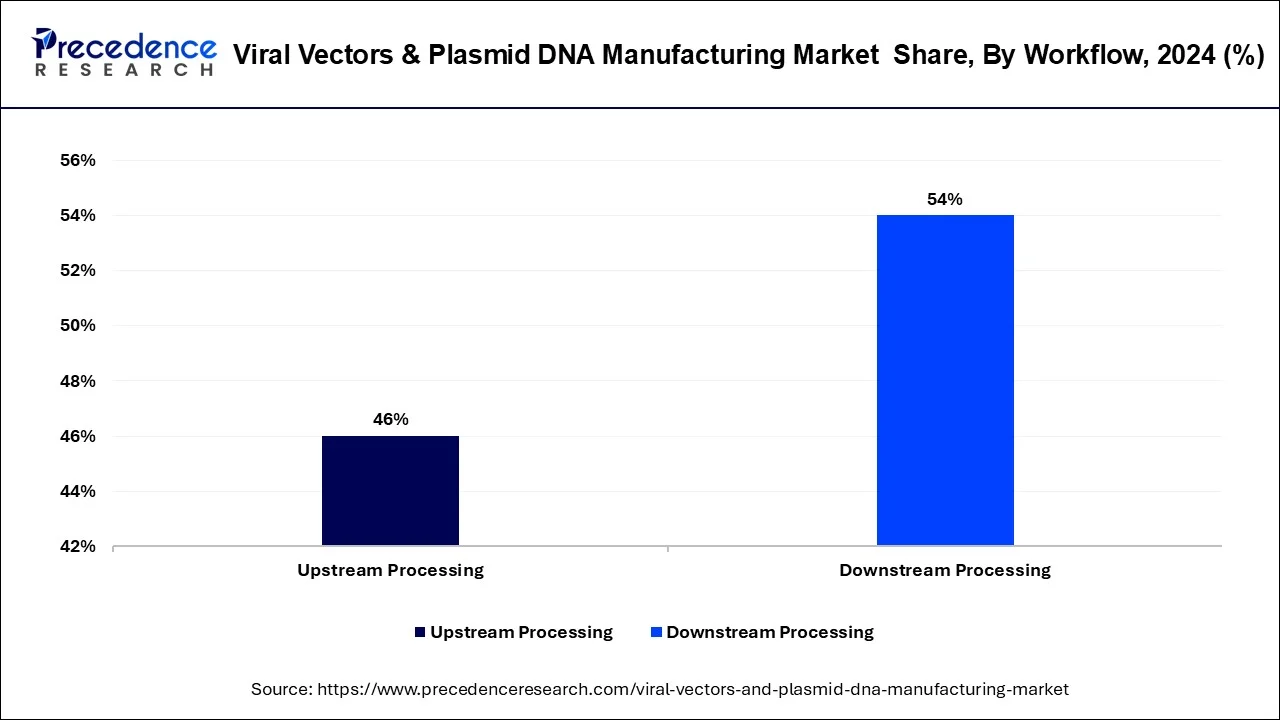
The downstream processing segment is the fastest-growing in the market during the forecast period, mainly on the basis of ensuring safety, purity, and efficacy of the finished therapeutic product. The activities of this step include purification, concentration, and formulation that make the product pure and compliant with specific regulatory requirements, including FDA and EMA. Technologies like chromatography, tangential flow filtration, and automation in purification are speeding up this growth by solving problems of scalability and cost.
End-User Analysis
The research institutes segment held the largest share in the viral vectors and plasmid DNA manufacturing market in 2024, owing to its fundamental functions in feed-forward research and development of advanced therapies. The core of innovation is in research institutes, which are involved in basic studies that research the properties of viral vectors and plasmid DNA, their safety, and performance. They have broad preclinical research, which paves the way to breakthroughs in gene therapy, vaccinology, and rare diseases.
The biopharmaceutical and pharmaceutical companies segment is expected to experience the fastest growth in the market during the forecast period, due to their aggressive investment plans and commercialization possibilities. Pharma and biotech companies are benefiting significantly from large funding and, highly developed manufacturing infrastructure. Such a combination of capital commitment, level of research and development, as well as focus on commercialization, makes them the fastest-growing end-user group in the market.
Disease Analysis
The genetic disorders segment dominated the viral vectors and plasmid DNA manufacturing market in 2024, mainly owing to the rising incidences of hereditary diseases like muscular dystrophy, cystic fibrosis, hemophilia, and other monogenic diseases. Demand has soared with the improved understanding of genetics, the successes of molecular biology, and increased approvals of gene therapies in rare and inherited diseases, and the development of new processes. Moreover, helpful government programs, patient lobbying, and industry investments have ensured fast innovation, with genetic disorders occupying the leadership position in the market.
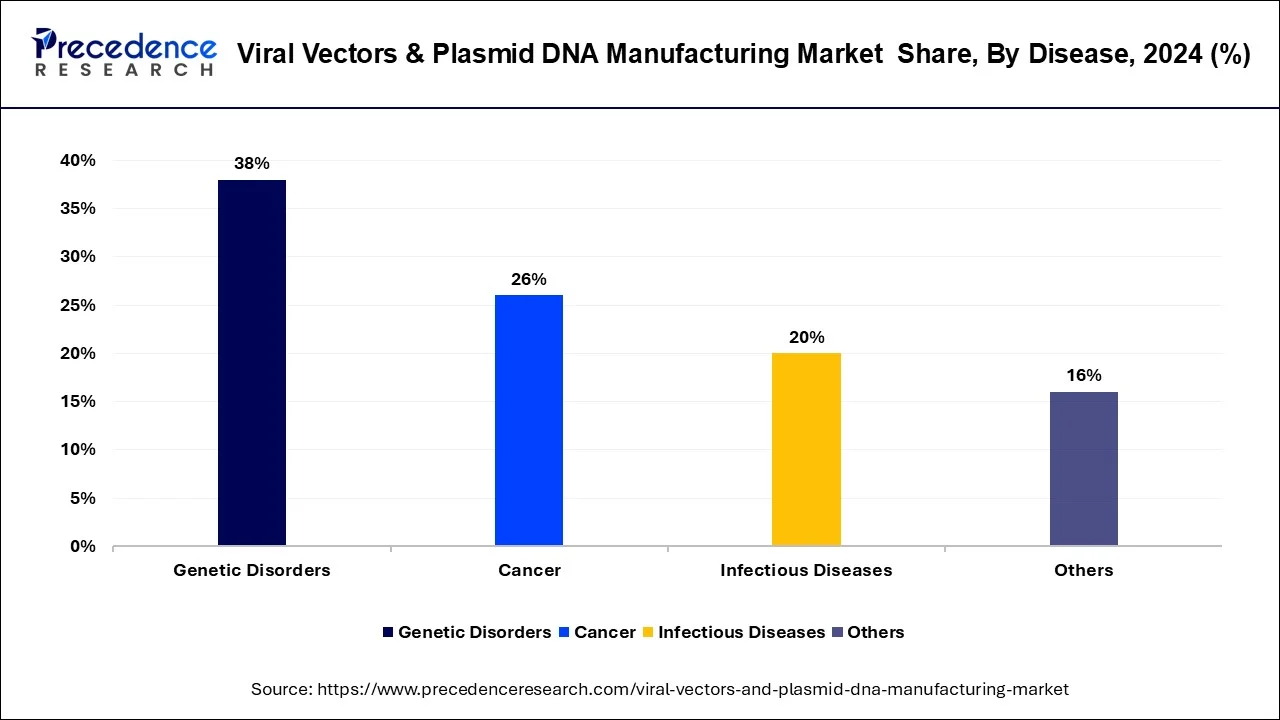
The cancer segment is the fastest-growing in the market during the forecast period, due to the rising usage of emerging therapies like CAR-T and oncolytic viral therapies. Delivery in the form of viral vectors and plasmid DNA is also important in allowing the targeted gene delivery to target immune cells or even attack tumors directly. The rise in global cancer rates and the urgent need for personalized and precision drug development are making pharmaceutical and biotech companies increase the production of vectors used as carriers in the oncology field.
Related Topics You May Find Useful:
➡️ Plasmid DNA Manufacturing Market: Explore how advanced therapies are shaping demand for high-quality plasmid DNA
➡️ U.S. Plasmid DNA Manufacturing Market: Analyze domestic capacity growth and biotech investment trends
➡️ Viral Vectors-Based Gene Therapy for Non-Human Primates Market: Track breakthroughs in preclinical models and translational research
➡️ Viral Vectors for Non-Human Primates Market: Understand how research is advancing in rare diseases and oncology applications
➡️ Adeno-Associated Virus Vector Manufacturing Market: Discover why AAV vectors dominate gene therapy pipelines
➡️ Cell and Gene Therapy CDMO Market: See how outsourcing is accelerating therapy development and scalability
➡️ Viral Vectors-Based Gene Therapy for Non-Human Primates Market: Explore the role of CDMOs in advancing viral vector platforms
Top Players in the Viral Vectors and Plasmid DNA Manufacturing Market
- Novasep
- Aldevron
- MerckWaismanBiomanufacturing
- Creative Biogene
- The Cell and Gene Therapy Catapult
- Cobra Biologics
- uniQure N.V.
- Addgene
- FUJIFILM Holdings Corporation
- Oxford Biomedicaplc
- Takara Bio Inc.
Latest Announcements by Industry Leaders
- A significant year for ongoing breakthroughs in cell and gene therapy (CGT) is 2024. A new era of solid tumor treatment with cell therapy has begun with the introduction of Amtagvi, a Tumor-Infiltrating Lymphocyte (TIL) therapy, on a global scale. Approving PM359, the first lead editing treatment in history, for clinical trials marks yet another advancement in gene editing technology. The development pipeline for CAR-T therapies will be significantly expanded with the upcoming clinical trials of INT2104, the first in vivo modified CAR-T therapy.
- In July 2024, The A-T Children’s Project is excited to host an international conference in November to address gene therapy for ataxia-telangiectasia (A-T). This conference will bring together clinicians, academic scientists, and industry leaders in the biotech and pharmaceutical fields who are pioneering DNA replacement and editing approaches.
Recent Developments:
- In August 2025, Probio launched its flagship U.S. plasmid DNA and viral vector GMP manufacturing facility. The site is constructed to support the end-to-end services to the gene and cell therapy developers with a size of 96,000 square feet. This plant served to boost the international operation Probio has as a CDMO and also offered greater scalability and faster production capability.
- In May 2025, CDMO 3PBIOVIAN executives indicate the company has since rolled out the AAVion platform, which is a complete integrated adeno-associated virus manufacturing platform aimed at speeding up the development of gene therapies. They further state that this model, the platform is built upon a proprietary HEK293 cell line, which integrates AAV manufacturing steps.
- In February 2023, BioNTech SE also reported that it had done the installation of the first plasmid DNA manufacturing facility in Germany. This has made the firm produce pDNA in-house to use clinically and commercially.
Viral Vectors and Plasmid DNA Manufacturing Market Segments Covered in the Report
By Vector Type
- Adenovirus
- Plasmid DNA
- Lentivirus
- Retrovirus
- AAV
- Others
By Application
- Gene Therapy
- Antisense &RNAi
- Cell Therapy
- Vaccinology
By Workflow
- Upstream Processing
- Vector Recovery/Harvesting
- Vector Amplification & Expansion
- Downstream Processing
- Fill-finish
- Purification
By End-User
- Biopharmaceutical and Pharmaceutical Companies
- Research Institutes
By Disease
- Genetic Disorders
- Cancer
- Infectious Diseases
- Others
By Regions
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Rest of the World
Thank you for reading. You can also get individual chapter-wise sections or region-wise report versions, such as North America, Europe, or Asia Pacific.
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/1012
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 804 441 9344
Stay Ahead with Precedence Research Subscriptions
Unlock exclusive access to powerful market intelligence, real-time data, and forward-looking insights, tailored to your business. From trend tracking to competitive analysis, our subscription plans keep you informed, agile, and ahead of the curve.
Browse Our Subscription Plans@ https://www.precedenceresearch.com/get-a-subscription
About Us
Precedence Research is a worldwide market research and consulting organization. We give an unmatched nature of offering to our customers present all around the globe across industry verticals. Precedence Research has expertise in giving deep-dive market insight along with market intelligence to our customers spread crosswise over various undertakings. We are obliged to serve our different client base present over the enterprises of medicinal services, healthcare, innovation, next-gen technologies, semi-conductors, chemicals, automotive, and aerospace & defense, among different ventures present globally.
Web: https://www.precedenceresearch.com
Our Trusted Data Partners:
Towards Healthcare | Towards Packaging | Towards Automotive | Towards Chem and Materials | Towards FnB | Towards Consumer Goods | Statifacts | Towards EV Solutions | Towards Dental | Nova One Advisor | Market Stats Insight
Get Recent News:
https://www.precedenceresearch.com/news
For the Latest Update Follow Us:
LinkedIn | Medium | Facebook | Twitter
✚ Explore More Market Intelligence from Precedence Research:
-
Pharmaceutical CDMO Market: Analyze how contract development boosts drug innovation and speed to market
-
U.S. Pharmaceutical CDMO Market: Track capacity expansion and regulatory advantages in the U.S. market
-
Topical Drugs CDMO Market: Discover rising opportunities in dermatology and transdermal drug delivery
-
Europe Pharmaceutical CDMO Market: Gain insights into regional strengths and growing demand in specialty drugs
-
Biologics CDMO Market: See how biologics outsourcing is scaling innovation in cell and gene therapies
-
Small Molecule Innovator CDMO Market: Understand shifts in demand for complex small-molecule development
-
Investigational New Drug CDMO Market: Explore how IND-focused CDMOs are shaping early-stage clinical success
-
Sterile Injectables CDMO Market: Analyze growth in high-value injectables and specialized manufacturing
-
Live Biotherapeutic Products and Microbiome CDMO Market: Discover emerging opportunities in microbiome-based therapies
-
mRNA Therapeutics CDMO Market: Track post-COVID demand for scalable mRNA platforms and vaccines
-
Pharmaceutical CDMO for Formulations Market: See how formulation expertise is driving drug stability and performance
-
Recombinant DNA Technology Market: Analyze innovations transforming genetic engineering and therapeutics
-
Targeted DNA RNA Sequencing Market: Discover how precision sequencing is advancing diagnostics and oncology
-
U.S. DNA Nanotechnology Market: Understand applications in drug delivery, biosensors, and molecular computing
-
U.S. Targeted DNA RNA Sequencing Market: Explore U.S.-based innovations in precision diagnostics and genomic medicine
- Pet DNA Testing Market: See how consumer interest is fueling growth in pet wellness and genetic testing

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

